Hydrogen index of acidity (pH). Instructions indicator paper - instructions litmus paper measure pH pH value
pH indicator and its impact on the quality of drinking water.
What is pH?
pH(“potentia hydrogeni” - the strength of hydrogen, or “pondus hydrogenii” - the weight of hydrogen) is a unit of measurement for the activity of hydrogen ions in any substance, quantitatively expressing its acidity.
This term appeared at the beginning of the twentieth century in Denmark. The pH indicator was introduced by the Danish chemist Soren Petr Lauritz Sorensen (1868-1939), although statements about a certain “power of water” are also found among his predecessors.
Hydrogen activity is defined as the negative decimal logarithm of the hydrogen ion concentration expressed in moles per liter:
pH = -log
For simplicity and convenience, the pH indicator was introduced in the calculations. pH is determined by the quantitative ratio of H+ and OH- ions in water, formed during the dissociation of water. It is customary to measure pH levels on a 14-digit scale.
If water has a reduced content of free hydrogen ions (pH greater than 7) compared to hydroxide ions [OH-], then the water will have alkaline reaction, and with an increased content of H+ ions (pH less than 7) - acid reaction. In perfectly pure distilled water, these ions will balance each other.
acidic environment: >
neutral environment: =
alkaline environment: >
When the concentrations of both types of ions in a solution are the same, the solution is said to be neutral. In neutral water the pH value is 7.
When dissolved in water, various chemical substances this balance changes, resulting in a change in pH value. When an acid is added to water, the concentration of hydrogen ions increases, and the concentration of hydroxide ions correspondingly decreases; when an alkali is added, on the contrary, the content of hydroxide ions increases, and the concentration of hydrogen ions decreases.
The pH indicator reflects the degree of acidity or alkalinity of the environment, while “acidity” and “alkalinity” characterize the quantitative content of substances in water that can neutralize alkalis and acids, respectively. As an analogy, we can give an example with temperature, which characterizes the degree of heating of a substance, but not the amount of heat. By putting our hand in the water, we can tell whether the water is cool or warm, but we will not be able to determine how much heat is in it (i.e., relatively speaking, how long this water will cool down).
pH is considered one of the most important indicators of drinking water quality. It shows the acid-base balance and influences how chemical and biological processes will proceed. Depending on the pH value, the flow rate may change chemical reactions, degree of corrosiveness of water, toxicity of pollutants, etc. Our well-being, mood and health directly depend on the acid-base balance of our body’s environment.
Modern man lives in a polluted environment. Many people purchase and consume food made from semi-finished products. In addition, almost every person is exposed to stress on a daily basis. All this affects the acid-base balance of the body's environment, shifting it towards acids. Tea, coffee, beer, carbonated drinks reduce pH in the body.
It is believed that an acidic environment is one of the main causes of cell destruction and tissue damage, the development of diseases and aging processes, and the growth of pathogens. In an acidic environment, building material does not reach the cells and the membrane is destroyed.
Externally, the state of the acid-base balance of a person’s blood can be judged by the color of his conjunctiva in the corners of his eyes. With an optimal acid-base balance, the color of the conjunctiva is bright pink, but if a person’s blood alkalinity increases, the conjunctiva becomes dark pink, and with an increase in acidity, the color of the conjunctiva becomes pale pink. Moreover, the color of the conjunctiva changes within 80 seconds after consuming substances that affect the acid-base balance.
The body regulates the pH of internal fluids, maintaining values at a certain level. The body's acid-base balance is a certain ratio of acids and alkalis that contributes to its normal functioning. The acid-base balance depends on maintaining relatively constant proportions between intercellular and intracellular waters in the tissues of the body. If the acid-base balance of fluids in the body is not constantly maintained, normal functioning and preservation of life will be impossible. Therefore, it is important to control what you consume.

Acid-base balance is our indicator of health. The more “sour” we are, the sooner we age and get sick. For the normal functioning of all internal organs, the pH level in the body must be alkaline, in the range from 7 to 9.
The pH inside our body is not always the same - some parts are more alkaline and some are acidic. The body regulates and maintains pH homeostasis only in certain cases, such as blood pH. The pH levels of the kidneys and other organs whose acid-base balance is not regulated by the body are affected by the food and drinks we consume.
Blood pH
The blood pH level is maintained by the body in the range of 7.35-7.45. The normal pH of human blood is considered to be 7.4-7.45. Even a slight deviation in this indicator affects the ability of the blood to carry oxygen. If the blood pH rises to 7.5, it carries 75% more oxygen. When the blood pH drops to 7.3, it is already difficult for a person to get out of bed. At 7.29, he can fall into a coma; if the blood pH drops below 7.1, the person dies.
Blood pH levels must be maintained within a healthy range, so the body uses organs and tissues to maintain a constant pH level. Because of this, the pH level of the blood does not change due to drinking alkaline or acidic water, but the tissues and organs of the body used to regulate the pH of the blood do change their pH.
Kidney pH
The pH parameter of the kidneys is influenced by water, food, and metabolic processes in the body. Acidic foods (such as meat products, dairy products, etc.) and drinks (sweetened carbonated drinks, alcoholic drinks, coffee, etc.) lead to low level pH in the kidneys because the body eliminates excess acidity through urine. The lower the urine pH level, the harder the kidneys have to work. Therefore, the acid load placed on the kidneys from such foods and drinks is called potential acid-renal load.
Drinking alkaline water benefits the kidneys - the urine pH level increases and the acid load on the body decreases. Increasing the pH of urine increases the pH of the body as a whole and rids the kidneys of acidic toxins.
Stomach pH
An empty stomach contains no more than a teaspoon of stomach acid produced at the last meal. The stomach produces acid as needed when eating food. The stomach does not produce acid when a person drinks water.
It is very useful to drink water on an empty stomach. The pH value increases to a level of 5-6. The increased pH will have a mild antacid effect and will lead to an increase in beneficial probiotics (good bacteria). Increasing the pH of the stomach increases the pH of the body, which leads to healthy digestion and relief from the symptoms of indigestion.
pH of subcutaneous fat
The body's fatty tissues have an acidic pH because excess acids are deposited in them. The body must store acid in fatty tissues when it cannot be excreted or neutralized by other means. Therefore, a shift in the body’s pH to the acidic side is one of the factors for excess weight.
The positive effect of alkaline water on body weight is that alkaline water helps remove excess acid from tissues because it helps the kidneys work more efficiently. This helps control weight because the amount of acid the body must “store” is greatly reduced. Alkaline water also improves the results of a healthy diet and exercise by helping the body deal with excess acidity produced by fat tissue during weight loss.
Bones
Bone has an alkaline pH because it is primarily composed of calcium. Their pH is constant, but if the blood needs pH adjustment, calcium is pulled from the bones.
The benefit of alkaline water to the bones is to protect them by reducing the amount of acid that the body has to fight against. Studies have shown that drinking alkaline water reduces bone resorption - osteoporosis.
Liver pH
The liver has a slightly alkaline pH, the level of which is affected by both food and drinks. Sugar and alcohol must be broken down in the liver, which leads to excess acid.
The benefits of alkaline water to the liver include the presence of antioxidants in such water; It has been found that alkaline water enhances the work of two antioxidants found in the liver, which contribute to more effective blood cleansing.
Body pH and alkaline water
Alkaline water allows the parts of the body that maintain the pH of the blood to function at greater efficiency. Increasing the pH levels in the parts of the body responsible for maintaining blood pH will help these organs stay healthy and functioning efficiently.
Between meals, you can help your body normalize its pH by drinking alkaline water. Even a small increase in pH can have a huge impact on your health.
According to research by Japanese scientists, the pH of drinking water, which is in the range of 7-8, increases the life expectancy of the population by 20-30%.
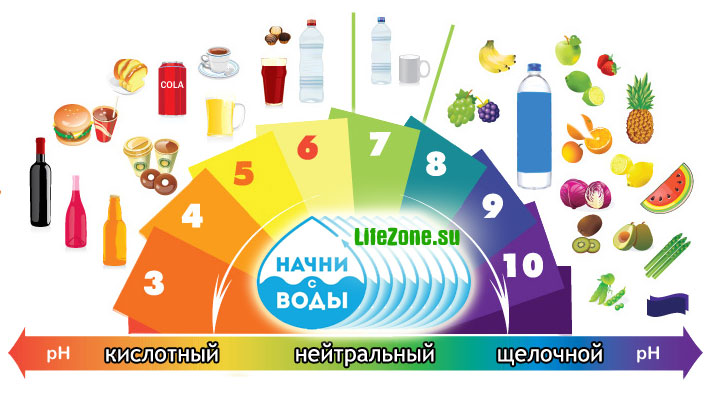
Depending on the pH level, water can be divided into several groups:
Strongly acidic waters< 3
acidic waters 3 - 5
slightly acidic waters 5 - 6.5
neutral waters 6.5 - 7.5
slightly alkaline waters 7.5 - 8.5
alkaline waters 8.5 – 9.5
highly alkaline waters > 9.5
Typically, the pH level of drinking tap water is within the range where it does not directly affect the consumer quality of water. IN river waters pH is usually in the range of 6.5-8.5, in precipitation 4.6-6.1, in swamps 5.5-6.0, in sea waters 7.9-8.3.
WHO does not offer any medically recommended value for pH. It is known that at low pH water is highly corrosive, and at high levels (pH>11) water acquires a characteristic soapiness, an unpleasant odor, and can cause irritation to the eyes and skin. That is why the optimal pH level for drinking and domestic water is considered to be in the range from 6 to 9.
|
|||||||||||||||||||||||||||||||||||||||||||||||



Interesting to know: The German biochemist OTTO WARBURG, awarded the Nobel Prize in Physiology or Medicine in 1931, proved that lack of oxygen (acidic pH<7.0) в тканях приводит к изменению нормальных клеток в злокачественные.
The scientist discovered that cancer cells lose the ability to develop in an environment saturated with free oxygen with a pH of 7.5 or higher! This means that when body fluids become acidic, cancer development is stimulated.
His followers in the 60s of the last century proved that any pathogenic flora loses the ability to reproduce at pH = 7.5 and above, and our immune system easily copes with any aggressors!
To preserve and maintain health, we need proper alkaline water (pH=7.5 and above). This will make it possible to better maintain the acid-base balance of body fluids, since the main living environments have a slightly alkaline reaction.
Already in a neutral biological environment, the body can have an amazing ability to self-heal.

Don't know where you can get it the right water ? I'll tell you!

Note:
Clicking the " To know"does not lead to any financial expenses or obligations.
You only get information about the availability of the right water in your region,
and get a unique opportunity to become a member of the healthy people club for free
and get a 20% discount on all offers + cumulative bonus.

Join the international health club Coral Club, receive a FREE discount card, the opportunity to participate in promotions, a cumulative bonus and other privileges!

In any solution is called acid-base equilibrium ( KShchR), although physiologists believe that it is more correct to call this ratio the acid-base state.
KSHR is characterized by a special pH indicator (power Hidrogen - “hydrogen power”), which shows the number of hydrogen atoms in a given solution.
At a pH of 7.0 they speak of a neutral environment.
The lower the pH level- those the environment is more acidic(from 6.9 to 0).
An alkaline environment has a high pH level (from 7.1 to 14.0).
The human body is on 70% consists of water, That's why water is one of its most important components.
The human body has a certain acid-base ratio, characterized by pH (hydrogen) value.
The pH value depends on the ratio between positively charged ions (forming an acidic environment) and negatively charged ions (forming an alkaline environment).
The body constantly strives to balance this ratio, maintaining a strictly defined pH level.
When the balance is disturbed, many serious diseases can occur.
 Check your acid-base balance with pH test strips.
Check your acid-base balance with pH test strips.
It is very important to pay attention in time to changes in the pH level of the internal environment of the body and, if necessary, take urgent measures.
Using pH test strips, you can easily, quickly and accurately determine your pH level without leaving your home.
The best time to determine the pH level is 1 hour before a meal or 2 hours after a meal.
Check the pH level 2 times a week 2-3 times a day.
Ignorance of your pH level can lead to dire consequences.
A) Increased acidity in the body
An imbalance in the pH of the body in most people manifests itself in the form of increased acidity (a condition Acidosis).
In this condition, the body poorly absorbs minerals such as calcium, sodium, potassium and magnesium, which, due to excess acidity, are excreted from the body.
Vital organs suffer from a lack of minerals.
If acidosis is not detected in time, it can harm the body unnoticed, but constantly for several months and even years.
Alcohol abuse often leads to acidosis.
Acidosis may occur as a complication of diabetes.
Acidosis may cause the following problems:
B) Increased alkali content in the body
With an increased content of alkali in the body, and this condition is called Alkalosis, just like with acidosis, the absorption of minerals is impaired.
Food is digested much more slowly, which allows toxins to pass from the gastrointestinal tract into the blood.
An increased level of alkali in the body is dangerous and difficult to correct.
As a rule, it is the result of the use of drugs containing alkali.
An increased alkali content can provoke:
Urine pH value
Urine pH test results show how well the body absorbs minerals such as calcium, sodium, potassium and magnesium.
These minerals are called "acid dampers" because they regulate the level of acidity in the body.
If the acidity is too high, the body does not produce acid.
It should neutralize the acid.
To do this, the body begins to borrow minerals from various organs, bones, etc. in order to neutralize excess acid that begins to accumulate in tissues.
Thus, the acidity level is regulated.
Minerals are used to neutralize acids
Over the course of 7 years, a study was conducted at the University of California (San Francisco), where 9 thousand women were examined.
The results showed that with constant elevated levels of acidity, bones become brittle.
The specialists who conducted this experiment are confident that most of the problems of middle-aged women are associated with excessive consumption of meat and lack of consumption of vegetable foods.
Therefore, the body has no choice but to take calcium from its own bones and use it to regulate the pH level.
(American Journal of Clinical Nutrition).
Saliva pH value

It is also rational to know the pH level of saliva.
Test results show the activity of enzymes in the digestive tract, especially the liver and stomach.
This indicator gives an idea of the work of both the entire organism as a whole and its individual systems.
Some people may have increased acidity in both urine and saliva - in which case we are dealing with "double acidity".
Blood pH value

Blood pH is one of the most stringent physiological constants in the body.
Normally, this indicator can vary within 7.3b - 7.42.
A shift in this indicator by even 0.1 can lead to severe pathology.
When the blood pH shifts by 0.2, a coma develops, and by 0.3, the person dies.
Maintain the correct pH balance for good health
The body is able to properly absorb and store minerals and nutrients only with the proper level of acid-base balance.
It is in your power to help your body receive, rather than lose, nutrients.
For example, iron Maybe assimilate body at pH 6,0 - 7,0 , A iodine- at pH 6,3 - 6,6 .
Our body uses hydrochloric acid to break down food.
In the process of vital activity of the body, both acidic and alkaline decomposition products are required, and 20 times more of the former are formed than the latter.
Therefore, the body’s defense systems, which ensure the invariability of its ASR, are “tuned” primarily to neutralize and remove, first of all, acidic decomposition products.
The main mechanisms for maintaining this balance are:
It is in your best interest to maintain the correct pH balance.
Even the “most correct” program for selecting medicinal herbs will not work effectively if your pH balance is imbalanced.
How the body manages acidity levels
Dietary supplement for normalizing ph balance in the body:
 Replenishes deficiencies of digestive enzymes Replenishes deficiencies of digestive enzymes Improves breakdown and absorption of nutrients Normalize the functioning of the digestive system Regulates the level of hydrochloric acid in the stomach Normalizes the microflora of the gastrointestinal tract Have an anti-inflammatory effect Regulate acid-base balance in the body 120 capsules | $21.56 |
By determining the pH of your saliva every day, you can monitor the condition of your body.
For identification of acids and bases.
For normal human metabolism, it is necessary that the acid-base balance be maintained within certain limits.
Solutions and liquids with respect to their acidity are considered:
neutral at pH = 7
acidic at pH< 7
alkaline at pH > 7
Acidity of urine
If the urine pH level fluctuates between 6.0 - 6.4 in the mornings and 6.4 - 7.0 in the evenings, then the body is functioning normally. The most optimal level is slightly sour, in the range of 6.4 - 6.5. A urine pH value below 5.0 indicates its sharp acidification, above 7.5 indicates its sharply alkaline reaction.
The reaction of urine determines the possibility of stone formation: rats - in an acidic environment, oxalate - in a neutral-acidic environment, phosphate - in a more alkaline environment. For example, uric acid stones virtually never occur when the urine pH is greater than 5.5, and phosphate stones never form unless the urine is alkaline. The best time to determine the pH level is 1 hour before or 2 hours after a meal.
Check the pH level twice a week 2-3 times a day.
Using indicator litmus paper pH test, you can easily, quickly and accurately monitor the response of urine to changes in the type of diet, the use of medications or dietary supplements. Positive pH dynamics can serve as a criterion for the correctness of the chosen diet or treatment.
The acidity of urine varies greatly depending on the food taken, for example, eating plant foods increases the alkaline reaction of urine. The acidity of urine increases if meat foods rich in proteins predominate in a person’s diet.
Heavy physical work increases the acidity of urine.
Increased acidity of urine is observed with increased acidity of the stomach. Reduced acidity of gastric juice does not affect the acidity of urine.
The acidity of urine changes in many diseases or conditions of the body, so determining its acidity is an important diagnostic factor.
Saliva acidity:
The acidity of saliva depends on the rate of salivation. Typically, the acidity of mixed human saliva is 6.8-7.4 pH, but with high salivation rates it reaches 7.8 pH. The acidity of the saliva of the parotid glands is 5.81 pH, and that of the submandibular glands is 6.39 pH. In children, the average acidity of mixed saliva is 7.32 pH.
Optimal measurement from 10 to 12 hours. It is better to measure it on an empty stomach, two hours before or two hours after a meal. Salivation decreases in the evening and at night.
To increase salivation, in order to increase the pH of saliva, it is good if there is a piece of lemon on the plate; it even with visual perception increases salivation. Food should look appetizing, served on beautiful dishes, appetizingly decorated with herbs and/or vegetables, it should, as they say, please the eye! Not only the saliva flows, but also the juices in the body, preparing for the process of digesting food. This is the mental phase of digestive secretion.
Acid gastroesophageal and pharyngolaryngeal refluxes reaching the oral cavity play a leading role in the occurrence of oral pathology. As a result of the ingress of hydrochloric acid, the acidity of mixed saliva decreases below 7.0 pH. Saliva, which normally has alkaline properties, at low pH, especially at values of 6.2-6.0, leads to focal demineralization of tooth enamel with the appearance of erosions of hard dental tissues and the formation of cavities in them - caries. The amount of mucus on the mucous membrane increases, the gums become swollen and inflamed.
When the acidity in the oral cavity decreases, the acidity of dental plaque decreases, which causes the development of caries.
Bacteria in the mouth thrive in the absence of air. Saliva, rich in oxygen, actively prevents their reproduction. Bad breath occurs when the flow of saliva slows down, for example during sleep. Excitement, hunger, pronouncing a long monologue, breathing through the mouth (for example, with a runny nose), stress - dry out the oral cavity, leading to a decrease in the pH of saliva. A decrease in saliva flow inevitably occurs with age.
You can use a slightly alkaline mouth rinse with water with the addition of soda and also take it orally between meals, proposed by Professor A.T. Ogulov. - slightly alkaline pH 7.4-8. Rinsing the mouth with soda water occurs for various inflammatory diseases of the gums and teeth and for general acidification of the body.
You can set the desired pH of water for rinsing or ingestion using litmus indicator paper. There cannot be recipes with the required proportions, because... Each region has its own water, with its own pH. Therefore, it is necessary to have indicator paper on hand.
Vaginal acidity
The normal acidity of a woman's vagina ranges from 3.8 to 4.4 pH and averages 4.0-4.2 pH.
Vaginal acidity in various diseases:
* cytolytic vaginosis: acidity less than 4.0 pH
* normal microflora: acidity from 4.0 to 4.5 pH
* candidal vaginitis: acidity from 4.0 to 4.5 pH
* Trichomonas colpitis: acidity from 5.0 to 6.0 pH
* bacterial vaginosis: acidity greater than 4.5 pH
* atrophic vaginitis: acidity greater than 6.0 pH
* aerobic vaginitis: acidity greater than 6.5 pH
Lactobacilli (lactobacillus) and, to a lesser extent, other representatives of normal microflora are responsible for maintaining an acidic environment and suppressing the growth of opportunistic microorganisms in the vagina. In the treatment of many gynecological diseases, restoration of the lactobacilli population and normal acidity comes to the fore.
Sperm acidity
The normal acidity level of sperm is between 7.2 and 8.0 pH. Deviations from these values are not in themselves considered pathology. At the same time, in combination with other deviations, it may indicate the presence of a disease.
An increase in the pH level of sperm occurs during an infectious process. A sharply alkaline reaction of sperm (acidity approximately 9.0-10.0 pH) indicates prostate pathology.
When the excretory ducts of both seminal vesicles are blocked, an acidic reaction of the sperm is observed (acidity 6.0-6.8 pH).
The fertilizing ability of such sperm is reduced. In an acidic environment, sperm lose motility and die. If the acidity of the seminal fluid becomes less than 6.0 pH, the sperm completely lose their motility and die.
Acidity of tears Normally - from 7.3 to 7.5 pH.
Acidity in the stomach. High and low acidity
The minimum theoretically possible acidity in the stomach is 0.86 pH.
The maximum theoretically possible acidity in the stomach is 8.3 pH.
Normal acidity in the lumen of the body of the stomach on an empty stomach is 1.5-2.0 pH.
The acidity on the surface of the epithelial layer facing the lumen of the stomach is 1.5-2.0 pH.
The acidity in the depths of the epithelial layer of the stomach is about 7.0 pH. Normal acidity in the antrum of the stomach is 1.3-7.4 pH.
The cause of many diseases of the digestive tract is an imbalance in the processes of acid production and acid neutralization. Long-term hypersecretion of hydrochloric acid or lack of acid neutralization, and, as a consequence, increased acidity in the stomach and/or duodenum, causes so-called acid-dependent diseases. Currently, these include: peptic ulcer of the stomach and duodenum, gastroesophageal reflux disease (GERD), erosive and ulcerative lesions of the stomach and duodenum while taking aspirin or non-steroidal anti-inflammatory drugs (NSAIDs), Zollinger-Ellison syndrome, gastritis and gastroduodenitis with high acidity and others.
Low acidity is observed with anacid or hypoacid gastritis or gastroduodenitis, as well as with stomach cancer. Gastritis (gastroduodenitis) is called anacid or gastritis (gastroduodenitis) with low acidity if the acidity in the body of the stomach is approximately 5 or more pH units. The cause of low acidity is often atrophy of parietal cells in the mucous membrane or disturbances in their functions.
Acidity in the intestines
Normal acidity in the duodenal bulb is 5.6-7.9 pH. The acidity in the jejunum and ileum is neutral or slightly alkaline and ranges from 7 to 8 pH. The acidity of small intestine juice is 7.2-7.5 pH. With increased secretion it reaches 8.6 pH. The acidity of the secretion of the duodenal glands is from pH 7 to 8 pH.
The acidity of pancreatic juice is from 7.5 to 9 pH.
The acidity of colon juice is 8.5-9.0 pH.
In the lower parts of the colon, pH values of acidity gradually increase, reaching a maximum pH value in the region of the rectosigmoid junction.
Acidityfeces Normally pH is from 6.0 to 8.0.
Acidity of meconium (original feces of newborns)- about 6 pH.
Acidity of human breast milk - 6.9-7.5 pH
Blood acidity
The acidity of human arterial blood plasma ranges from 7.37 to 7.43 pH, averaging 7.4 pH. The acid-base balance in human blood is one of the most stable parameters, maintaining acidic and alkaline components in a certain balance within very narrow limits. Even a small shift from these limits can lead to severe pathology. When shifting to the acidic side, a condition called acidosis occurs, and to the alkaline side, alkolosis occurs. A change in blood acidity above 7.8 pH or below 6.8 pH is incompatible with life.
The acidity of red blood cells is 7.28-7.29 pH.
Normal blood revitalizes lymph cells that can destroy tumor cells. There are many lymphatic cells in the human body (eg, NK cells, LAK cells). Their uniqueness lies in the fact that they are able to distinguish normal cells from diseased and damaged ones, and destroy the latter. This is the function of the human body's immunity. The greatest activity of lymphatic cells in destroying diseased cells occurs at pH 7.4. However, usually around the affected cells, there is a more acidic environment, which interferes with the activity of lymphocytes, which work better at a slightly alkaline pH. By consuming foods that have an alkalizing effect, you can adjust the pH balance within 0.5 units, creating a favorable environment for the action of lymphocytes and the destruction of affected or abnormally constructed cells.
Cancerous tissue has increased acidity, unlike normal tissue, and the body protects it with a fibrous membrane whose pH is alkaline. If you continue to follow the acidic diet, the membrane dissolves and the cancer cells are released.
Once a week, when the body is acidified, it is advisable to arrange healing days for yourself, eating only vegetables (1.5 kg of vegetables, divided throughout the day), boiled and sometimes raw in the summer, only heat-treated in the autumn-winter) and Be sure to have clean hot water.
Equally important for maintaining normal levelspH The body also has a person’s mood; a good, cheerful mood normalizes the acid-base balance. Laugh more!
ATTENTION! When copying text or part of the text, a mandatory link to the site
The hydrogen index - pH - is a measure of the activity (in the case of dilute solutions, reflects the concentration) of hydrogen ions in a solution, quantitatively expressing its acidity, calculated as the negative (taken with the opposite sign) decimal logarithm of the activity of hydrogen ions, expressed in moles per liter.
pH = – log
This concept was introduced in 1909 by the Danish chemist Sørensen. The indicator is called pH, after the first letters of the Latin words potentia hydrogeni - the strength of hydrogen, or pondus hydrogenii - the weight of hydrogen.
The inverse pH value is somewhat less widespread - an indicator of the basicity of the solution, pOH, equal to the negative decimal logarithm of the concentration of OH ions in the solution:
рОН = – log
In pure water at 25°C, the concentrations of hydrogen ions () and hydroxide ions () are the same and amount to 10 -7 mol/l, this directly follows from the autoprotolysis constant of water K w, which is otherwise called the ionic product of water:
K w = =10 –14 [mol 2 /l 2 ] (at 25°C)
pH + pH = 14
When the concentrations of both types of ions in a solution are the same, the solution is said to be neutral. When an acid is added to water, the concentration of hydrogen ions increases, and the concentration of hydroxide ions correspondingly decreases; when a base is added, on the contrary, the content of hydroxide ions increases, and the concentration of hydrogen ions decreases. When > the solution is said to be acidic, and when > it is alkaline.
pH determination
Several methods are widely used to determine the pH value of solutions.
1) The pH value can be approximately estimated using indicators, accurately measured with a pH meter, or determined analytically by performing acid-base titration.
To roughly estimate the concentration of hydrogen ions, acid-base indicators are widely used - organic dye substances, the color of which depends on the pH of the medium. The most well-known indicators include litmus, phenolphthalein, methyl orange (methyl orange) and others. Indicators can exist in two differently colored forms - either acidic or basic. The color change of each indicator occurs in its own acidity range, usually 1-2 units (see Table 1, lesson 2).
To expand the working range of pH measurements, a so-called universal indicator is used, which is a mixture of several indicators. The universal indicator changes color sequentially from red through yellow, green, blue to violet when moving from an acidic region to an alkaline one. Determining pH by the indicator method is difficult for cloudy or colored solutions.
2) The analytical volumetric method - acid-base titration - also gives accurate results for determining the total acidity of solutions. A solution of known concentration (titrant) is added dropwise to the test solution. When they are mixed, a chemical reaction occurs. The equivalence point - the moment when there is exactly enough titrant to completely complete the reaction - is recorded using an indicator. Next, knowing the concentration and volume of the added titrant solution, the total acidity of the solution is calculated.
The acidity of the environment is important for many chemical processes, and the possibility or outcome of a particular reaction often depends on the pH of the environment. To maintain a certain pH value in the reaction system during laboratory research or in production, buffer solutions are used, which allow maintaining an almost constant pH value when diluted or when small amounts of acid or alkali are added to the solution.
The pH value is widely used to characterize the acid-base properties of various biological media (Table 2).
The acidity of the reaction medium is of particular importance for biochemical reactions occurring in living systems. The concentration of hydrogen ions in a solution often affects physicochemical characteristics and the biological activity of proteins and nucleic acids, therefore, for the normal functioning of the body, maintaining acid-base homeostasis is a task of exceptional importance. Dynamic maintenance of the optimal pH of biological fluids is achieved through the action of buffer systems.
3) The use of a special device - a pH meter - allows you to measure pH in a wider range and more accurately (up to 0.01 pH units) than using indicators, is convenient and highly accurate, allows you to measure the pH of opaque and colored solutions and therefore widely used.
Using a pH meter, the concentration of hydrogen ions (pH) is measured in solutions, drinking water, food products and raw materials, environmental objects and production systems for continuous monitoring of technological processes, including in aggressive environments.
A pH meter is indispensable for hardware monitoring of pH solutions for the separation of uranium and plutonium, when the requirements for the correctness of equipment readings without calibration are extremely high.
The device can be used in stationary and mobile laboratories, including field laboratories, as well as clinical diagnostic, forensic, research, and production laboratories, including the meat, dairy and baking industries.
Recently, pH meters are also widely used in aquarium farms, monitoring water quality in domestic conditions, agriculture (especially in hydroponics), and also for monitoring health diagnostics.
Table 2. pH values for some biological systems and other solutions
|
System (solution) | |
|
Duodenum | |
|
Gastric juice | |
|
Human blood | |
|
Muscle | |
|
Pancreatic juice | |
|
Protoplasm of cells | |
|
Small intestine | |
|
Sea water | |
|
Chicken egg white | |
|
Orange juice | |
|
Tomato juice | |
Temporarily out of stock
Expected arrival
Litmus paper (pH test) 80 strips from 1 to 14 pH Article: 309
Pack of 80 litmus strips for pH tests. The packaging contains a pH color chart.
Measurement step - 1
With the help of pH test strips, you can determine the acid-base balance (pH) of any liquid medium without leaving your home. Our pH tests have the widest measurement range, namely from 1 to 14, and are suitable for both medical and household needs, for example:
To determine the quality of milk,
To determine the acidity of water, urine,
When checking pH for aquarium and swimming pools,
When monitoring drinking, natural and other waters.
Directions for use: immerse the test strip into the sample being tested.
solution for 1-2 seconds, then remove it and compare after 15 seconds
coloring an indicator strip with a color scale.
The scale is represented by the following values: 1 2 3 4 5 6 7 8 9 10 11 12 13 14
The pH level, also called the hydrogen index, characterizes the concentration of hydrogen ions. In a neutral environment, the pH value is 7, in acidic environments it is less than 7, and in alkaline environments it is above 7
Manufacturer country: China

